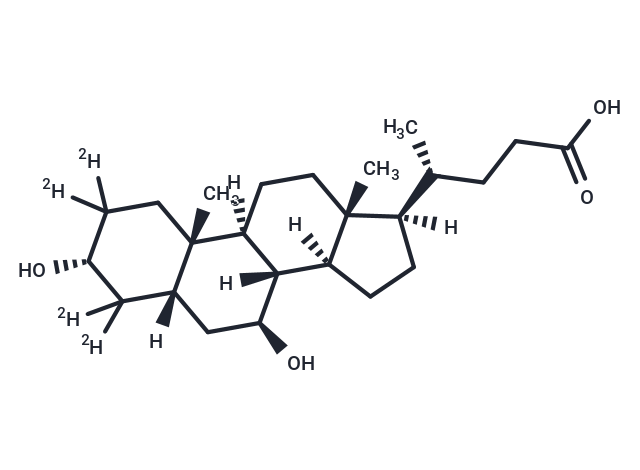
Ursodeoxycholic Acid-d4
CAS No. 347841-46-7
Ursodeoxycholic Acid-d4( —— )
Catalog No. M37724 CAS No. 347841-46-7
Ursodeoxycholic Acid-d4 (Ursodeoxycholic acid-2,2,4,4-d4) is the deuterium substitute of Ursodeoxycholic acid, and is an internal standard for the quantitative determination of Ursodeoxycholic Acid (UDCA) levels by LC/MS or GC/MS.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 2MG | 160 | Get Quote |


|
| 5MG | 217 | Get Quote |


|
| 10MG | 325 | Get Quote |


|
| 25MG | 583 | Get Quote |


|
| 50MG | 984 | Get Quote |


|
| 500MG | Get Quote | Get Quote |


|
| 1G | Get Quote | Get Quote |


|
Biological Information
-
Product NameUrsodeoxycholic Acid-d4
-
NoteResearch use only, not for human use.
-
Brief DescriptionUrsodeoxycholic Acid-d4 (Ursodeoxycholic acid-2,2,4,4-d4) is the deuterium substitute of Ursodeoxycholic acid, and is an internal standard for the quantitative determination of Ursodeoxycholic Acid (UDCA) levels by LC/MS or GC/MS.
-
DescriptionUrsodeoxycholic acid-2,2,4,4-d4 is the deuterium labeled Ursodeoxycholic acid (HY-13771). Ursodeoxycholic acid is a secondary bile acid issued from the transformation of (cheno)deoxycholic acid by intestinal bacteria, acting as a key regulator of the intestinal barrier integrity and essential for lipid metabolism. Ursodeoxycholic acid acts as signaling molecule, exerting its effects by interacting with bile acid activated receptors, including G-protein coupled bile acid receptor 5 (TGR5, GPCR19) and the farnesoid X receptor (FXR). Ursodeoxycholic acid can be used for the research of a variety of hepatic and gastrointestinal diseases. Ursodeoxycholic acid also reduces ACE2 expression and is beneficial for reducing SARS-CoV-2 infection.
-
In VitroStable heavy isotopes of hydrogen, carbon, and other elements have been incorporated into drug molecules, largely as tracers for quantitation during the drug development process. Deuteration has gained attention because of its potential to affect the pharmacokinetic and metabolic profiles of drugs.
-
In Vivo——
-
Synonyms——
-
PathwayOthers
-
TargetOther Targets
-
RecptorOthers
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number347841-46-7
-
Formula Weight396.6
-
Molecular FormulaC24H40O4
-
Purity>98% (HPLC)
-
Solubility——
-
SMILESC[C@@]12[C@]([C@]3([C@@]([C@]4(C)[C@](C[C@@H]3O)(C([C@H](O)C(C4)([2H])[2H])([2H])[2H])[H])(CC1)[H])[H])(CC[C@@]2([C@@H](CCC(O)=O)C)[H])[H]
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference



-
Necroptosis-IN-3
Necroptosis-IN-3 (Cyclohexanecarboxamide, N-(2-thienylmethyl)-) (Compound 69) is a Necroptosis inhibitor that inhibits TNF-α induced necroptosis. Necroptosis-IN-3 (Compound STX1638) also inhibits 11β-HSD1.
-
Benzoylaconitine
Benzoylaconine is an alkaloid in the Chinese traditional medicine Radix Aconiti Lateralis Preparata (Fuzi). Benzoylaconine and aconitine can induce reproductive toxicity in BeWo Cell the amino acid metabolism was the main metabolic pathway and responsible for the placental and fetal toxicity of them.
-
[Ala286]-Calmodulin-...
[Ala286]-Calmodulin-Dependent Protein Kinase II (281-302) is a modified fragment of calcium/calmodulin-dependent protein kinase II that contains the active domain of CaMKII and has an alanine substitution at position 286. [Ala286]-Calmodulin-Dependent Protein Kinase II (281-302) can be used to develop more potent CaMKII inhibitors.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


